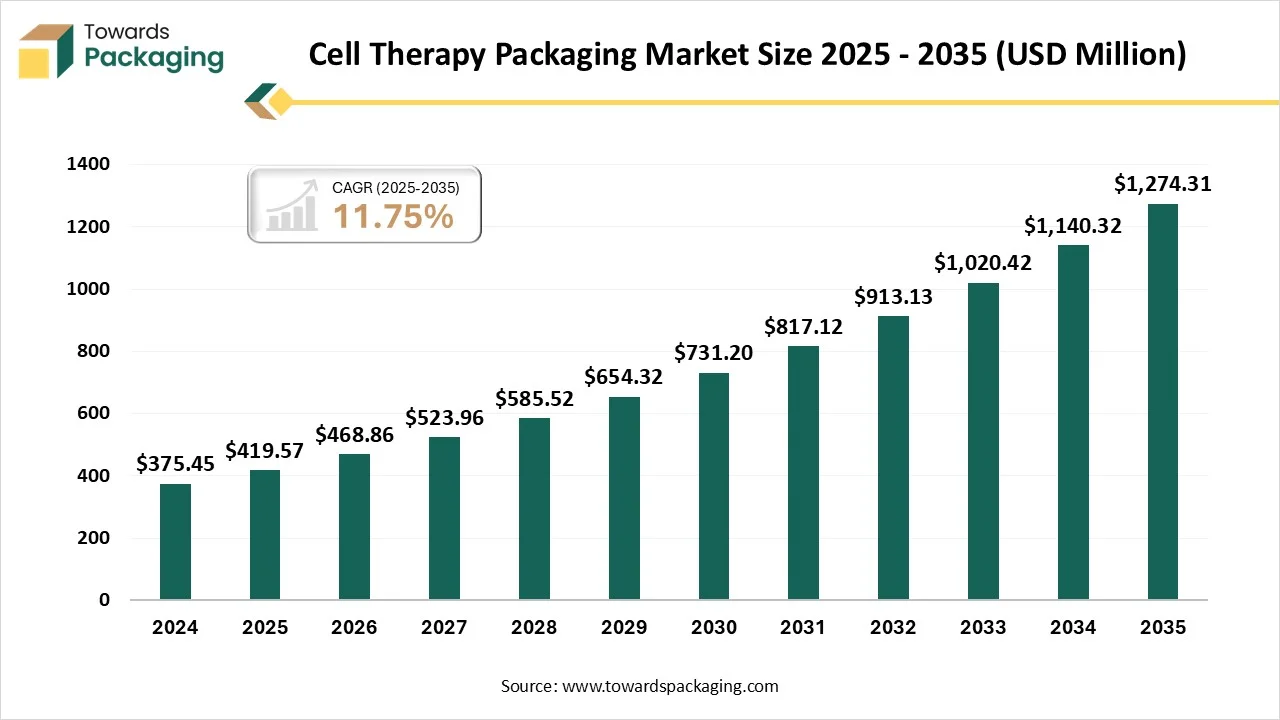
Ottawa, Nov. 28, 2025 (GLOBE NEWSWIRE) -- The global cell therapy packaging market reported a value of USD 419.57 million in 2025, and according to estimates, it will reach USD 1274.31 million by 2034, as outlined in a study from Towards Packaging, a sister firm of Precedence Research. Cell therapy packaging is significant because it ensures the quality, sterility, and stability of complex biological products via specialized, temperature-controlled containment and even transport solutions.
Request Research Report Built Around Your Goals: sales@towardspackaging.com
What is Meant by Cell Therapy Packaging?
Cell therapy packaging mainly refers to the specialized, multi-layered system programmed to protect and transport live cells utilized in treatments, ensuring their viability and even stability throughout the cold chain. It includes primary containers such as cryobags or vials that directly hold the cells, secondary packaging such as racks or boxes for organization, and thus, tertiary packaging like dry vapor shippers, which maintain cryogenic temperatures during transport.
The main drivers for cell therapy packaging are the growing demand for cell and gene therapies, the complexity of such products requiring specific conditions for storage along with transport, and technological developments that enable more precise and even effective packaging solutions. Its significance extends to protecting the high-value nature of these therapies from physical damage, contamination, and even temperature fluctuations, and ensuring the drug's viability from production to patient administration.
Government Initiatives for Cell Therapy Packaging:
- US FDA's Regulatory Framework for Cellular & Gene Therapy (CGT) Products: The FDA regulates most cell and gene therapy products as biologics under the Public Health Service (PHS) Act and Federal Food, Drug, and Cosmetic Act, requiring manufacturers to validate packaging systems (primary, secondary, and tertiary) to maintain product stability and closure integrity during extreme cryogenic storage and shipping.
- Indian DBT's National Biopharma Mission (NBM) for GMP-grade Manufacturing: The Department of Biotechnology (DBT) in India supports projects aimed at establishing Good Manufacturing Practices (GMP) facilities for critical components like viral vectors, which indirectly drives the need for standardized, compliant packaging solutions for the resultant cell therapies.
- EU's Packaging and Packaging Waste Regulation (PPWR): This regulation, while general, establishes overarching rules for all packaging in the EU, including requirements for minimal packaging weight/volume, recyclability, and the phasing out of hazardous substances like certain PFAS, which applies to cell therapy logistics where materials must be safe and environmentally compliant.
- Development of Indigenous CAR-T Cell Therapy in India: Government support through the DBT and BIRAC for India's first home-grown, affordable CAR-T therapy, "NexCAR19," includes ensuring the entire supply chain, including packaging, meets rigorous national and international standards to guarantee safety and efficacy from lab to patient.
Get All the Details in Our Solutions - Access Report Sample: https://www.towardspackaging.com/download-sample/5873
What are the Latest Trends in the Cell Therapy Packaging Market?
- Development of Specialized Packaging for Specific Cell Types like T-Cells and Stem Cells
These therapies have unique and even strict stability requirements that standard packaging cannot meet, like precise temperature control along with sterile environments. Developing specialized packaging addresses the specific needs of these cell types, guaranteeing their viability and efficacy during transport and storage, which is vital for their success as a therapeutic product. This trend is driven by the rising complexity and sensitivity of advanced cell therapies and the demand to maintain their quality and safety.
What Potentiates the Growth of the Cell Therapy Packaging Market?
- Rising Demand and Increasing Approvals for Novel Cell and Gene Therapies
The rising prevalence of chronic and rare diseases, mainly cancer and autoimmune disorders, has heightened the demand for advanced, usually personalized, treatment options such as T-cell and stem cell therapies.
Regional Analysis
Who is the Leader in the Cell Therapy Packaging Market?
North America leads the market due to a combination of a strong, well-established pharmaceutical industry, significant investment in research and development, supportive regulatory environments, and a large number of commercialized as well as pipeline therapies. Meanwhile, the U.S. Food and Drug Administration (FDA) have initiatives such as Regenerative Medicine Advanced Therapy (RMAT) along with Breakthrough Therapy designations which accelerate the development and even approval of novel therapies.
U.S. Cell Therapy Packaging Market Trends
The U.S. market is rising rapidly, driven by the increasing need for cell therapies, mainly T-cell therapies for oncology, and also advancements in gene editing technologies. Key trends involve a shift towards the commercial scale segment, expansion in allogeneic therapies, and the incorporation of automation, digitalization, and AI for manufacturing and quality control.
Canada Market Trends
Canada's market is undergoing significant expansion, driven by the increasing need for autologous therapies and solid government support for production. Key trends involve a major focus on scalable manufacturing as well as technologies for cell biology, mainly autologous therapies, with the market anticipated to grow substantially in the coming years.
How is the Opportunistic Rise of Asia Pacific in the Cell Therapy Packaging Market?
The Asia-Pacific is a global framework for cell therapy research, with China alone hosting over 50% of all cell therapy clinical trials globally. This extensive research pipeline, mainly in oncology and even regenerative medicine, directly fuels the need for specialized cell therapy packaging solutions that ensure product stability and integrity. Thus, the integration of automated and even scalable manufacturing along with packaging solutions is a key trend, allowing firms to improve efficiency and decrease the risk of contamination, which is vital for the complex logistics of cell therapies.
China Cell Therapy Packaging Market Trends
Key trends in China's market involve rapid growth driven by strong government support and even a high volume of clinical trials, a shift towards commercial-scale production packaging, along with an increasing target on autologous and T-cell therapies, which need specialized and also advanced packaging solutions. The market is also seeing international collaboration to speed up manufacturing and meet demand.
India Market Trends
India's market is undergoing robust growth, driven by the rising adoption of autologous therapies, mainly in CAR-T cell therapy, which is funded by domestic innovations and government initiatives. Key trends involve the commercial scale segment's faster expansion compared to clinical scale, a strong target on primary packaging solutions, and the growing demand for eco-friendly and even advanced packaging materials to thus meet environmental standards and patient needs.
More Insights of Towards Packaging:
- Custom Sustainable Boxes for E-Commerce Market Size, Trends, Segmentation, and Regional Analysis 2025-2034
- Plant-Based Plastics Market Size, Trends, Segments, and Regional Outlook 2025-2035
- Decor Paper Market Size, Trends, Segmentation & Global Opportunity Analysis (2025-2035)
- Ovenable Paperboard Trays Market Size, Trends, Segments, Companies, Competitive Analysis, Value Chain & Trade Analysis 2025-2035
- Gift Wrapping Paper Market Size, Trends, Segments, Companies, Competitive Analysis, Value Chain & Trade Analysis 2025-2035
- Kraft Paper Bag Market Size, Competitive Analysis, Value Chain & Trade Analysis 2025-2034
- Molded Paper Pulp Packaging Market Size, Trends, and Regional Insights 2025-2035
- Uncoated Recycled Paperboard Market Size, Trends, Key Segments, and Regional Dynamics with Manufacturers and Suppliers Data
- Paper Bag Packaging Market Size, Trends, and Forecast Analysis (2025-2034): Regional Insights, Market Segments, and Competitive Dynamics
- Single-Use Plastic Water Bottles Market Size, Trends, Segments, Companies, Competitive Analysis, Value Chain & Trade Analysis 2026-2035
- PET Blow Molder Market Size, Trends, Key Segments, and Regional Dynamics with Manufacturers and Suppliers Data
- U.S. Beverage Packaging Market Size, Trends, Key Segments, and Regional Dynamics with Manufacturers and Suppliers Data
- High-Barrier Packaging Films Market Size, Trends, Key Segments, and Regional Dynamics with Manufacturers and Suppliers Data
- Leak-Proof Flexible Packaging Market Size, Trends, Segments, Regional Outlook (NA, EU, APAC, LA, MEA), Competitive Landscape, Value Chain & Trade Analysis 2025-2035
- Glass-to-Plastic Packaging Market Size, Trends, Segments, Regional Analysis, Trade Insights & Competitive Landscape
Segment Outlook
Packaging Type Insights
Why did the Primary Packaging Segment Dominate the Cell Therapy Packaging Market in 2024?
It is the first layer of protection that is in direct contact with the therapy, guaranteeing its sterility, efficacy, and even stability. Its importance is driven by the demand to preserve the sensitive nature of cell therapies, control dosage, and even offer essential information directly on the product itself. Primary packaging, like pre-filled syringes, is usually customized for specific therapies, which assists ensure accurate dosage for personalized treatments and also reduces the chance of medication errors.
The tertiary packaging segment is the fastest-growing in the market during the forecast period. Due to the increasing demand for secure, temperature-controlled, and also bulk-handling logistics to fund the complex supply chain of cell therapies, which includes innovations in shipping containers, pallets, and even automated warehousing. Its growth is also propelled by the booming e-commerce sector and even rising need for sustainable solutions.
Rising environmental concerns and regulations are driving a need for more sustainable packaging alternatives, including recyclable as well as biodegradable materials for tertiary packaging solutions.
Material Type Insights
Why did the Cryogenic Plastics Segment Dominate the Cell Therapy Packaging Market in 2024?
Cryogenic plastics are vital for managing the ultra-cold temperatures needed by cell therapies, which are usually highly sensitive to temperature fluctuations. The growing commercialization of personalized cell-driven treatments, like for cancer and rare diseases, fuels the required for specialized packaging which can manage strict cold chain requirements.
The single-use polymer segment is the fastest-growing in the market during the forecast period. Cell therapies are greatly susceptible to contamination, and removing cross-contamination risk is paramount, mainly for patient-specific (autologous) therapies, where no second batch of material is available. Further, single-use systems (SUS) are pre-sterilized and engineered as closed systems, lowering exposure to the external environment and thus dramatically reducing contamination risks and even ensuring product integrity. By decreasing the time required for setup, cleaning, changeovers, and validation, single-use technologies speed up the overall production timeline and also speed up a product's time to market
Function Insights
Why did the Storage Packaging Segment dominate the Cell Therapy Packaging Market in 2024?
Due to the fundamental demand to preserve cell viability throughout the supply chain, mainly given the sensitive nature of thus, these high-value products. The need is driven by the rising number of therapies demanding specific temperature controls, ranging from cryogenic to refrigerated to ambient, that necessitate a wide variety of specialized, high-integrity storage solutions.
The transportation packaging segment is the fastest-growing in the market during the forecast period. Due to the rising number of complexes, temperature-sensitive cell therapies, and even the resulting high need for specialized and even cold-chain logistics solutions. This demands robust, temperature-controlled transport packaging to manage the viability of these advanced therapies during global distribution, from the production site to the patient. Cell therapies are extremely sensitive to temperature fluctuations along require rigorous cold chain management to guarantee their safety and efficacy.
Cell Therapy Type Insights
Why did the CAR-T Cell Therapies Segment Dominate the Cell Therapy Packaging Market in 2024?
Due to its absolute success in treating blood cancers, solid investment, regulatory approvals, as well as a strong pipeline of new therapies, it is anticipated to be a major player in the field. CAR-T therapy has showed high remission and even durability rates in treating few blood cancers, like leukemia and lymphoma, stating a strong track record of success where conventional therapies have faltered. Improvements in gene editing technologies, like CRISPR, are leading to more efficient, precise, and safer CAR-T therapies, thus improving their attractiveness.
The stem cell therapies segment is the fastest-growing in the market during the forecast period. Due to the high potential of stem cells to treat a wide range of diseases, especially chronic and degenerative conditions, and even significant research, funding, and development are underway in the field. Developments in iPSC technology, as well as other stem cell research, are opening up new possibilities for advancing therapies and for drug discovery.
End User Insights
Why did the Cell Therapy Manufacturers / Biopharma Companies Segment dominate the Cell Therapy Packaging Market in 2024?
Due to high need from their successful and rising product pipelines, mainly for CAR-T cell therapies, which treat blood cancers with high efficacy as well as low relapse rates. As more goods advance toward commercialization, there is an increasing need for large-scale, reliable, and cost-effective production and packaging solutions.
The specialized logistics & cryo-transport companies segment is considered as the fastest growing in the market during the forecast period. These therapies are highly sensitive to time as well as temperature and need specialized expertise and infrastructure that conventional logistics providers lack. The rapid growth of the cell therapy clinical pipeline, together with commercial approvals, has increased the need for these specific services. The transportation, along with the storage of cell therapies, is subject to rigorous regulations (e.g., FDA, EMA, GMP, GDP guidelines).
Specialized logistics manufacturers have the expertise to guarantee strict compliance, includes meticulous documentation for the chain of identity (COI) and chain of custody (COC), lowering the risk of product loss or mix-ups which can have fatal consequences for patients.
Elevate your packaging strategy with Towards Packaging. Enhance efficiency and achieve superior results - schedule a call today: https://www.towardspackaging.com/schedule-meeting
Recent Breakthroughs in the Global Cell Therapy Packaging Industry
- In January 2023, Catalent, the leader in enabling the development as well as supply of better treatments for patients globally, declared the launch of its new Case Management Service, which has now been specifically designed to tackle the unique challenges linked with the safe and timely delivery of advanced therapies to patients by offering professional supply chain oversight from program start to finish.
Top Companies in the Cell Therapy Packaging Market & Their Offerings
- West Pharmaceutical Services: Provides primary containment like Daikyo Crystal Zenith® vials for cryogenic storage.
- SCHOTT AG: Offers high-quality glass and polymer vials and syringes for safe storage of sensitive drugs.
- Catalent Pharma Solutions: Provides end-to-end services including packaging, cold chain storage, and distribution for cell therapies.
- Avantor, Inc.: Supplies workflow components and single-use solutions for manufacturing, not final physical packaging containers.
- DWK Life Sciences: Manufactures RTU glass/plastic vials and closure systems for pharmaceutical applications.
- ArcticZyme Technologies ASA: Focuses on enzymes for biomanufacturing processes, not cell therapy packaging.
- Cold Chain Technologies LLC: Provides insulated thermal shippers (e.g., CCT TheraShield) for rigorous temperature requirements.
- Sonoco ThermoSafe: Offers passive/active temperature-controlled packaging solutions and logistics for life sciences transport.
- CSafe Global: A leading provider of passive, active, and specific cell/gene therapy cold chain shipping solutions.
- BioLife Solutions, Inc.: Offers biopreservation media and cryogenic storage vials designed to maintain cell viability.
- Intelsius Ltd.: Designs and manufactures validated insulated containers and temperature-controlled shippers.
- CryoXpert GmbH: Focuses on specialized services and equipment for the cryogenic supply chain logistics.
- Stirling Ultracold: Manufactures ultra-low temperature (ULT) freezers for on-site storage of biological materials.
Segments Covered in the Report
By Packaging Type
- Primary Packaging (Cryovials, Cryobags, Cell Containers)
- Secondary Packaging (Protective Sleeves, Rigid Boxes, Insulated Holders)
- Tertiary Packaging (Shipping Systems, Cryogenic Shippers, Overpacks)
- Ancillary Components (Labels, Caps, Seals, Tamper-Proof Devices)
By Material Type
- Cryogenic Plastics (Polypropylene, Polycarbonate, HDPE)
- Glass (Cryovials, Ampoules)
- Metal & Composite Alloys (Liquid Nitrogen Shippers, Racks)
- Single-Use Polymer Systems (Multilayer Films, EVA Bags)
- Paperboard & Corrugated Materials (Outer Packaging)
By Function
- Storage Packaging (Cryogenic Freezing & Long-term Storage)
- Transportation Packaging (Cold Chain & Cryogenic Shipping)
- Primary Containment & Labeling Systems
- Safety & Contamination Control Packaging
- Regulatory & Serialization Packaging Solutions
By Cell Therapy Type
- CAR-T Cell Therapies
- Stem Cell Therapies (MSC, iPSC, HSC)
- NK Cell Therapies
- Dendritic Cell Therapies
- Others (Adoptive T-cell, Gene-modified Cells)
By End User
- Cell Therapy Manufacturers / Biopharma Companies
- Contract Manufacturing & Development Organizations (CDMOs / CMOs)
- Academic & Research Institutes
- Specialized Logistics & Cryo-Transport Companies
- Hospitals & Transplant Centers
By Region
- North America:
- U.S.
- Canada
- Mexico
- Rest of North America
- South America:
- Brazil
- Argentina
- Rest of South America
- Europe:
- Western Europe
- Germany
- Italy
- France
- Netherlands
- Spain
- Portugal
- Belgium
- Ireland
- UK
- Iceland
- Switzerland
- Poland
- Rest of Western Europe
- Western Europe
- Eastern Europe
- Austria
- Russia & Belarus
- Türkiye
- Albania
- Rest of Eastern Europe
- Asia Pacific:
- China
- Taiwan
- India
- Japan
- Australia and New Zealand,
- ASEAN Countries (Singapore, Malaysia)
- South Korea
- Rest of APAC
- MEA:
- GCC Countries
- Saudi Arabia
- United Arab Emirates (UAE)
- Qatar
- Kuwait
- Oman
- Bahrain
- South Africa
- Egypt
- Rest of MEA
Invest in Our Premium Strategic Solution: https://www.towardspackaging.com/checkout/5873
Request Research Report Built Around Your Goals: sales@towardspackaging.com
About Us
Towards Packaging is a global consulting and market intelligence firm specializing in strategic research across key packaging segments including sustainable, flexible, smart, biodegradable, and recycled packaging. We empower businesses with actionable insights, trend analysis, and data-driven strategies. Our experienced consultants use advanced research methodologies to help companies of all sizes navigate market shifts, identify growth opportunities, and stay competitive in the global packaging industry.
Stay Connected with Towards Packaging:
- Find us on Social Platforms: LinkedIn | Twitter | Instagram | Threads
- Subscribe to Our Newsletter: Towards Sustainable Packaging
- Visit Towards Packaging for In-depth Market Insights: Towards Packaging
- Read Our Printed Chronicle: Packaging Web Wire
- Get ahead of the trends – follow us for exclusive insights and industry updates:
Pinterest | Medium | Tumblr | Hashnode | Bloglovin | LinkedIn – Packaging Web Wire | Globbook | Substack | Bluesky | - Contact: APAC: +91 9356 9282 04 | Europe: +44 778 256 0738 | North America: +1 8044 4193 44
Our Trusted Data Partners
Precedence Research | Statifacts | Towards Healthcare | Towards Auto | Towards Food and Beverages | Towards Chemical and Materials | Towards Consumer Goods | Towards Dental | Towards EV Solutions | Nova One Advisor | Healthcare Webwire | Packaging Webwire | Automotive Webwire | Nutraceuticals Func Foods | Onco Quant | Sustainability Quant | Specialty Chemicals Analytics
Towards Packaging Releases Its Latest Insight - Check It Out:
- Cling Films Market Size, Trends, Segments, Regional Outlook, Competitive Landscape & Trade Statistics
- Edible Boxes Market Size, Trends, Segmentation, Regional Outlook, Competitive Landscape & Trade Statistics Report
- Food and Beverage Packaging Materials Market Size, Growth Trends, Segments, Regional Insights, Trade Statistics & Competitive Landscape Analysis
- Trolley Bags Market Size, Trends, Segments, Regional Insights & Competitive Landscape Report
- Recycled Polyethylene Terephthalate (rPET) Market Size, Trends, Segments, Regional Insights, Companies, Competitive Landscape, Value Chain & EU–APAC Trade Data Analysis
- Plastic Blister Packs Market Size, Trends, Key Segments, and Regional Dynamics with Manufacturers and Suppliers Data
- Expanded Polystyrene for Packaging Market Size, Trends, Segments, Companies, Competitive Analysis, Value Chain & Trade Analysis 2025-2035
- Circular Packaging Market Size, Share, Trends, Segmentation, and Regional Analysis 2025-2035
- Polyhydroxyalkanoates Films Market Size, Trends, Segmentation, and Global Regional Analysis 2025-2035
- PFAS-Free Food Packaging Market Size, Share, Trends, Segmentation, and Regional Analysis 2025-2035
- Recycled Packaging for Apparel Market Size, Share, Trends, Segments, and Regional Analysis 2025-2035
- Closed-Loop Packaging Systems Market Size, Share, Trends, Segments, and Regional Analysis 2025-2035
- PHA Bioplastics Market Size, Trends, Segments, and Regional Insights 2025-2035
- Blockchain-Integrated Smart Packaging Market Size, Growth Trends, and Industry Segmentation 2025-2035
- Carbon-Negative Packaging Market Size, Trends, and Global Industry Analysis 2025-2035



